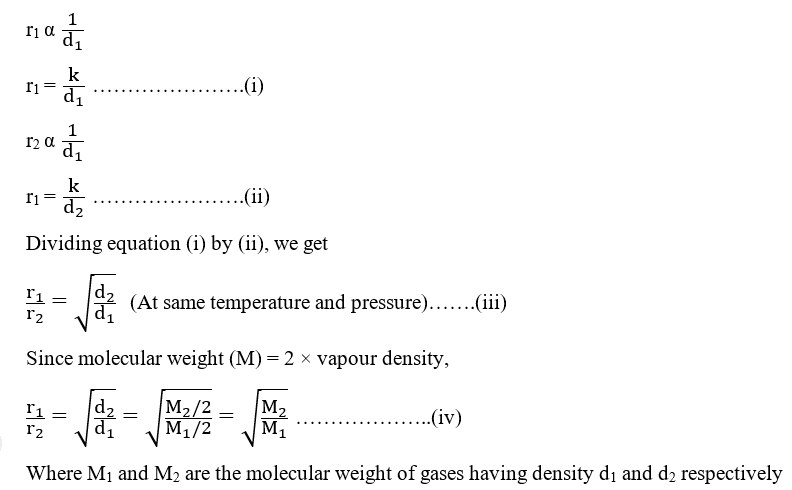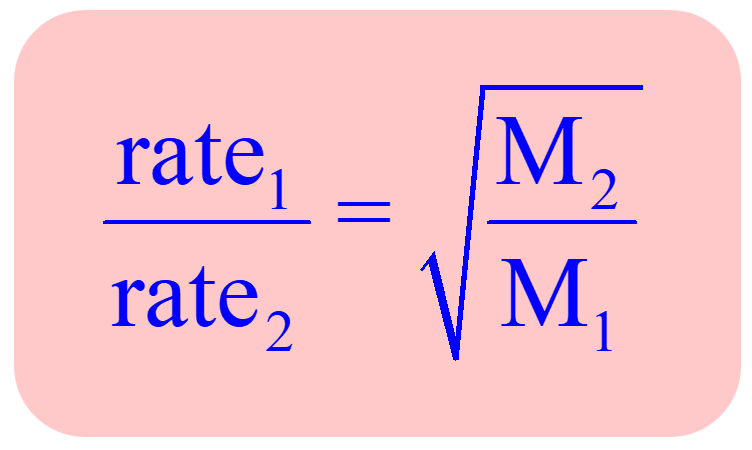Can the rate of effusion or diffusion be negative, in accordance with Graham's law? If so, how? - Quora
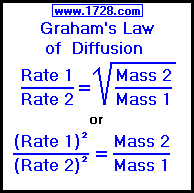
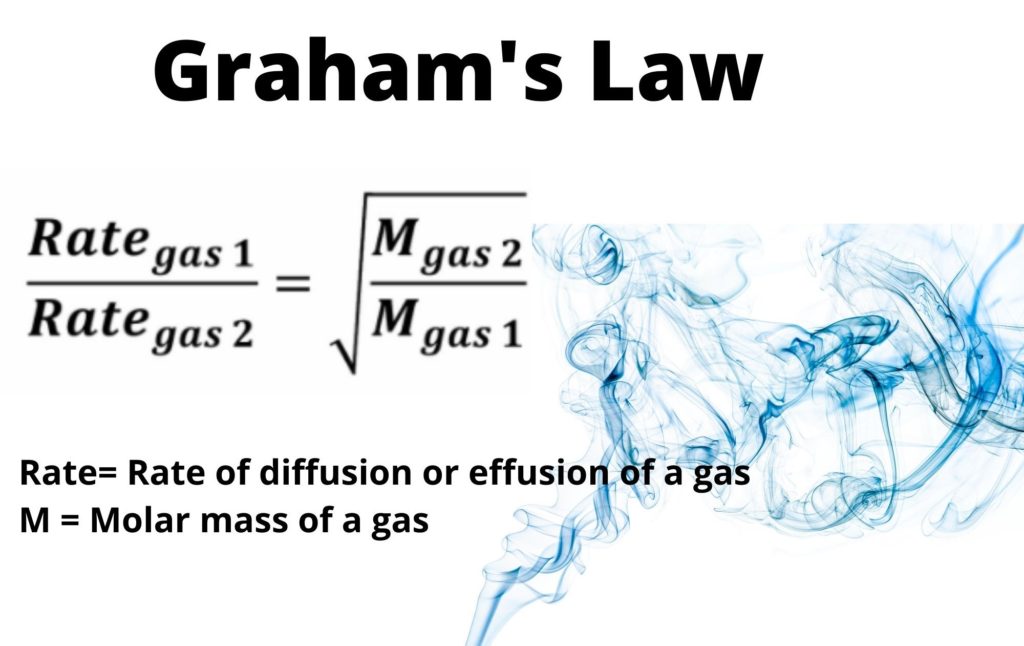
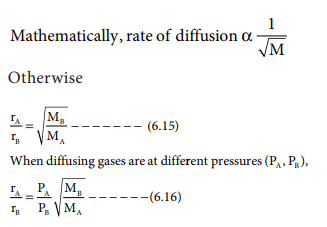
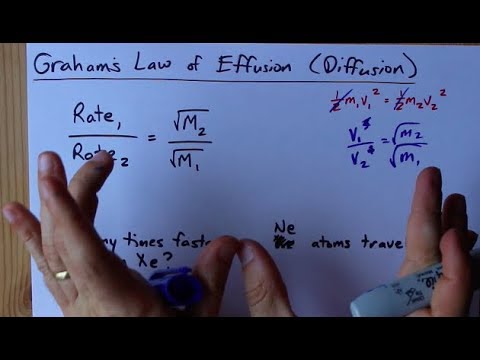
Rate of diffusion of a saturated hydrocarbon is about 1/6 th of that of hydrogen under similar conditions of temperature and pressure. What is the molecular formula of that hydrocarbon?
Can the rate of effusion or diffusion be negative, in accordance with Graham's law? If so, how? - Quora