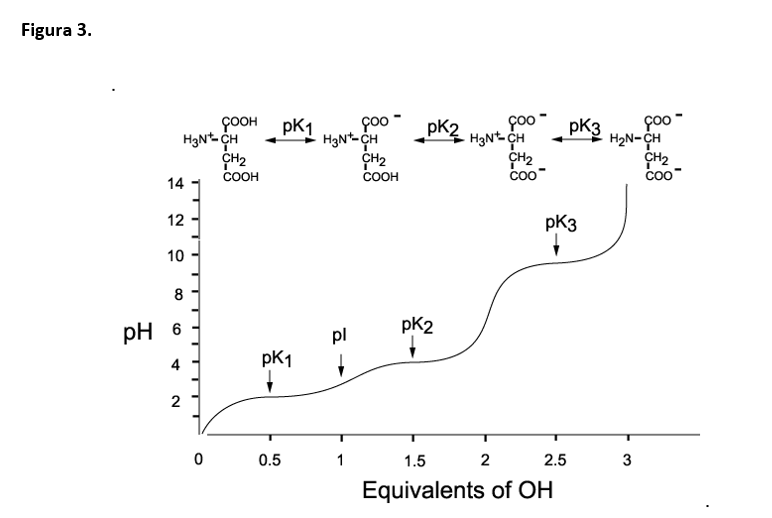
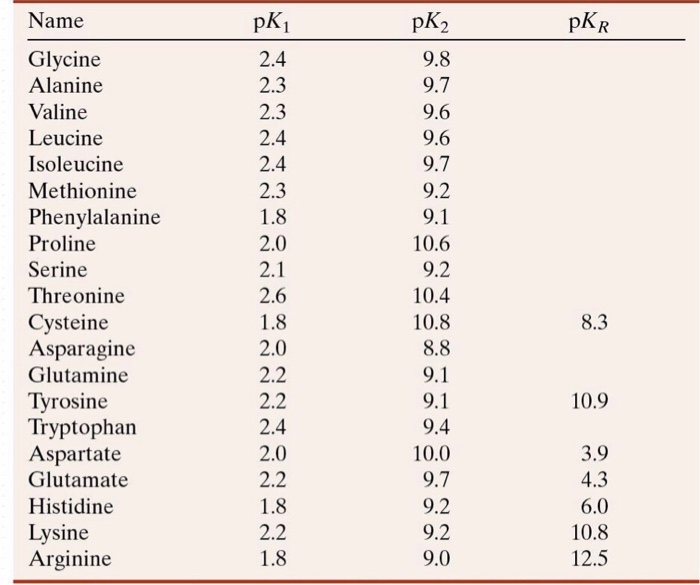
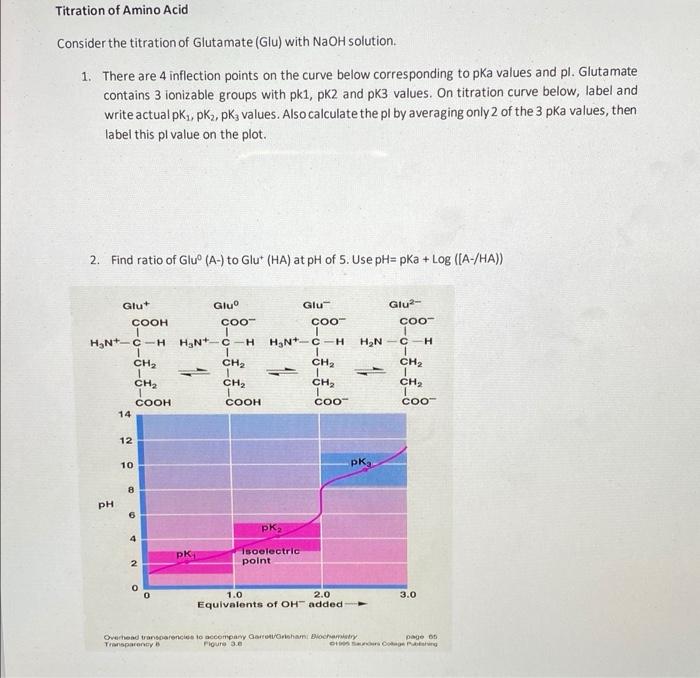
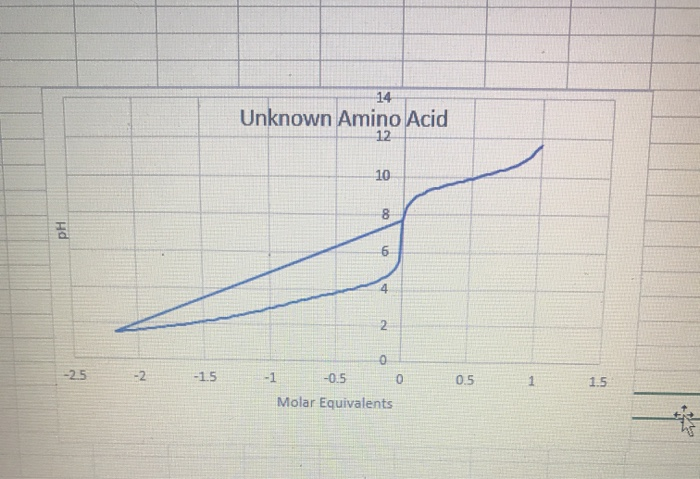
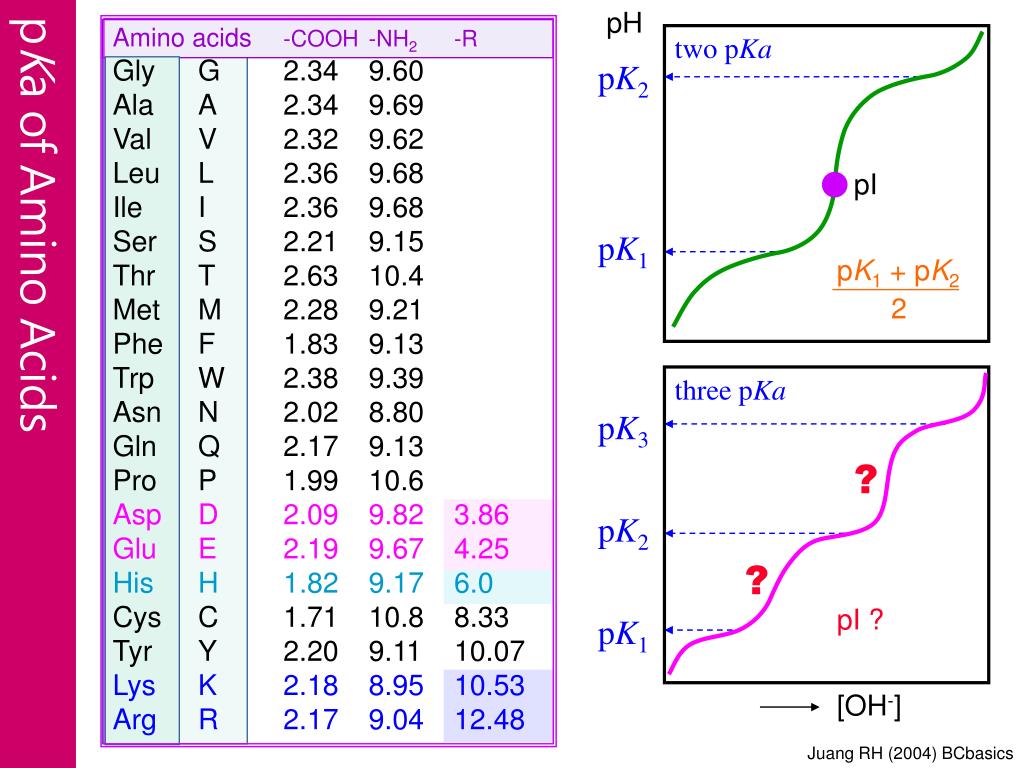
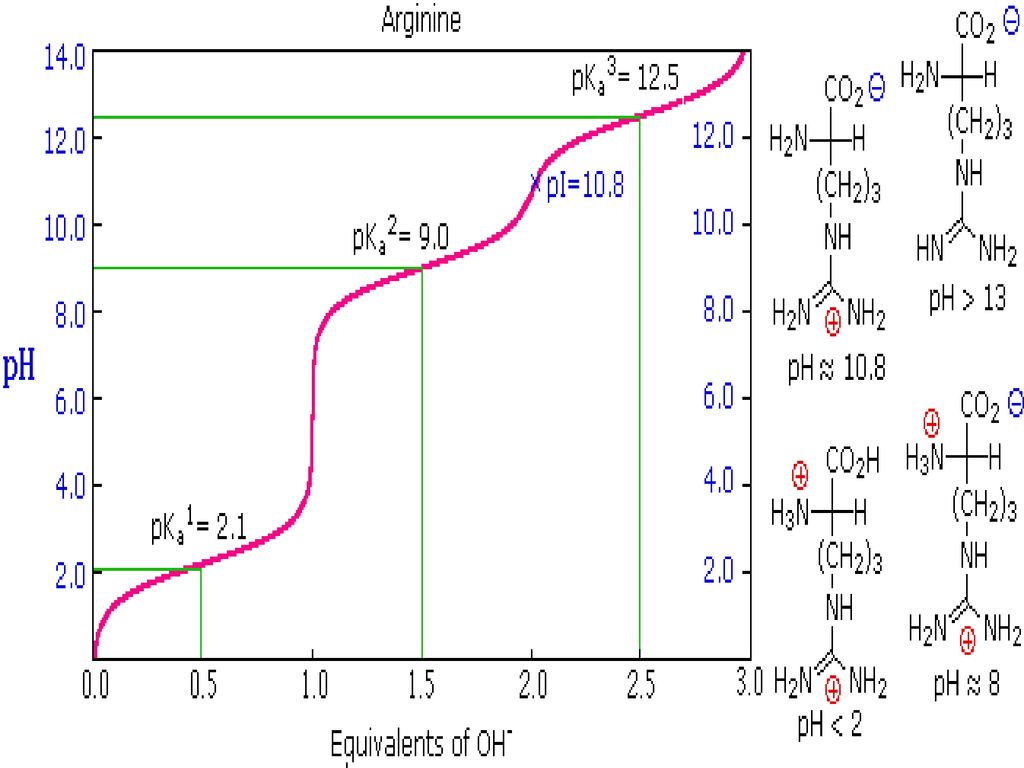
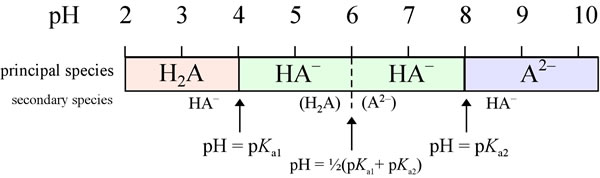
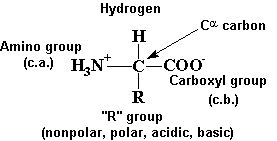BC367 Experiment 1 Identification of an Unknown Amino Acid Introduction As the building blocks of proteins, amino acids play a k

Scheme of synthesis for DEAE–CH derivatives; pK1, pK2 and pK3 indicate... | Download Scientific Diagram
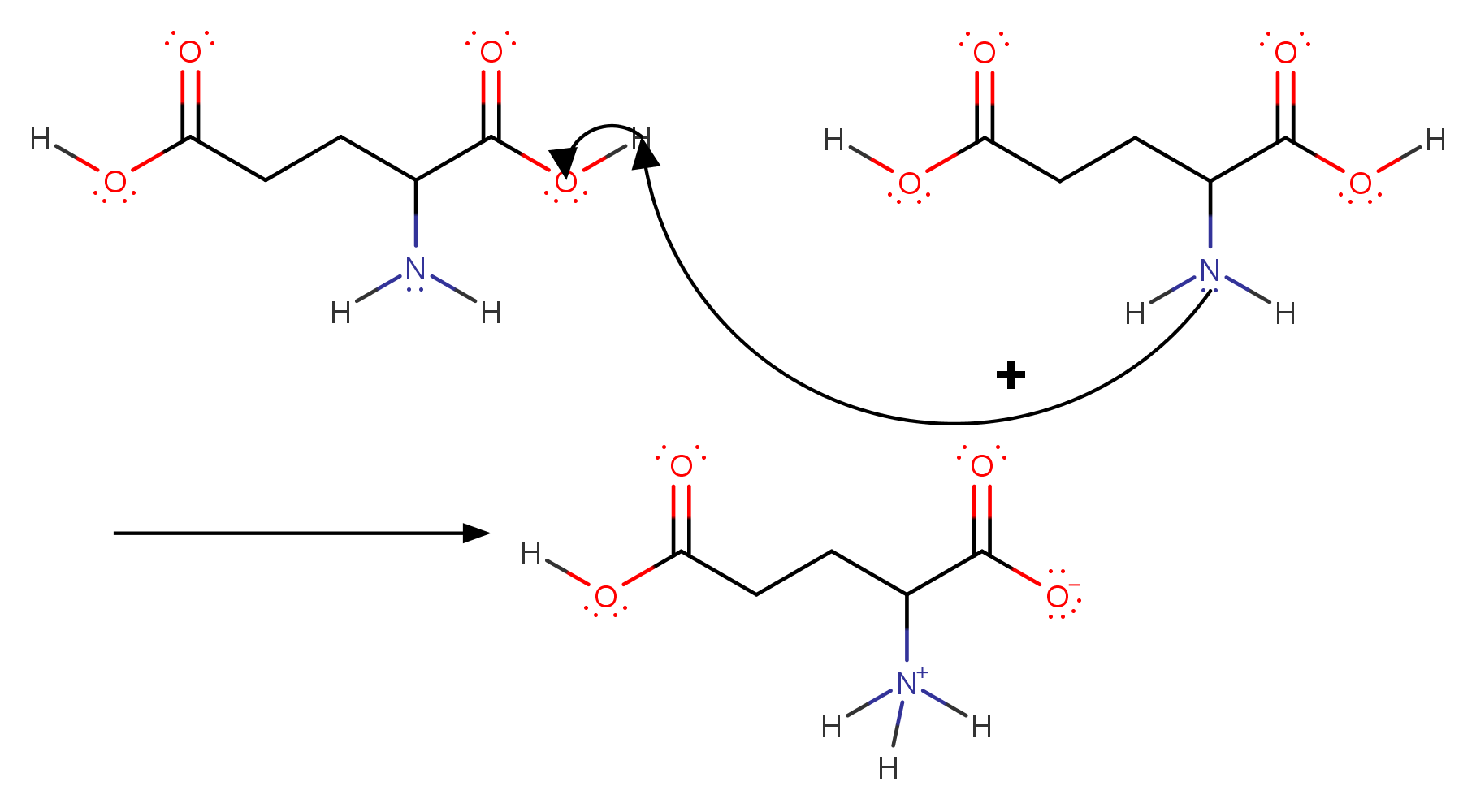
Using the Pka' Values for glutamic acid (pk1= 2.19, pk2= 9.67, pkR= 4.25) indicate the ionic form which predominates at: a) pH 1.0 b)pH 7.0 c) pH13 d) What is the net
PROPERTIES OF AMINO ACIDS A. Isomerism: Two types of isomerism are shown by amino acids basically due to the presence of asymmet
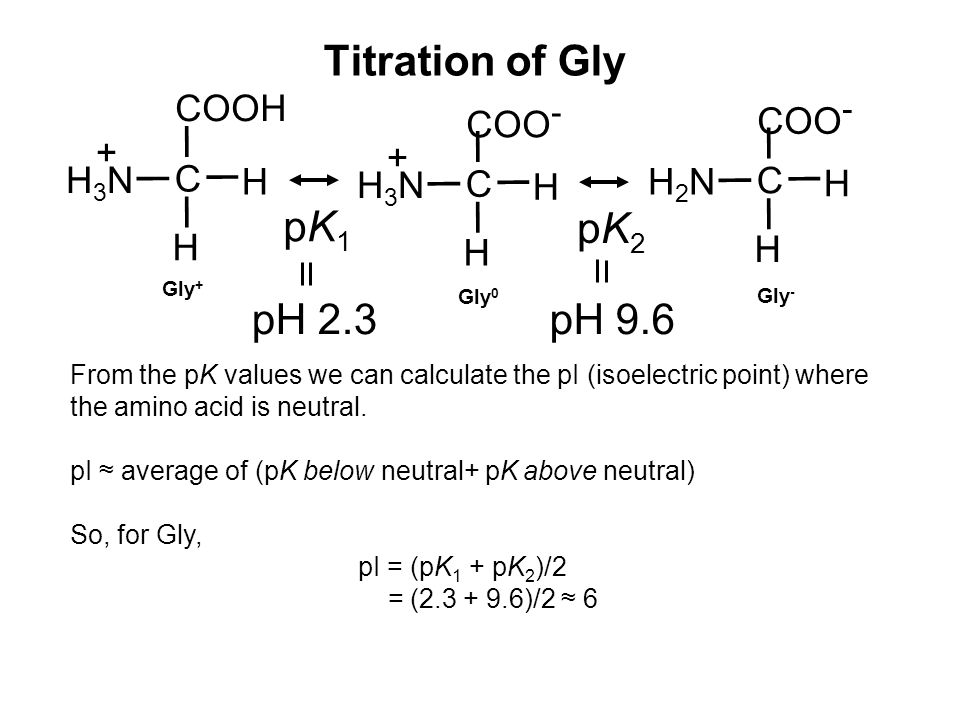
Lecture 3: Amino Acids Bonus seminar today at 3PM 148 Baker (bonus point assignment due on Wed. in class or electronically by ) Quiz next Wed. (9/7) - ppt video online download

The amino acid methionine has pKa1 = 2.2 and pKa2 = 9.1. If this amino acid is represented by H2L+, what is the major species at pH 6? | Homework.Study.com
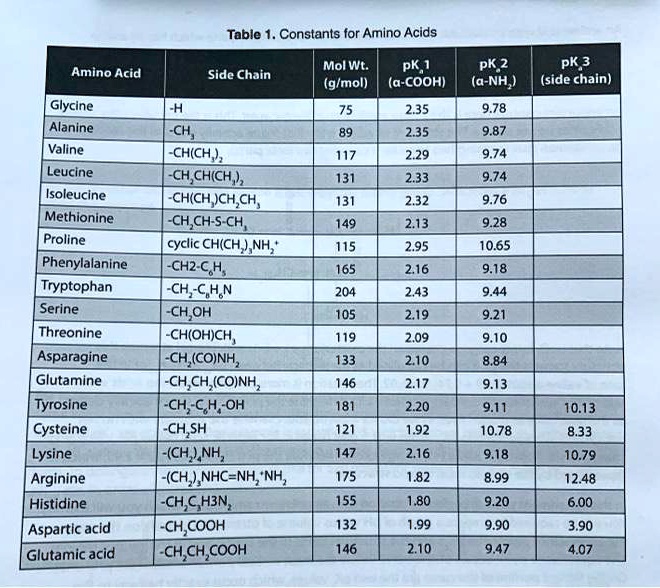
Table Constants for Amino Acids Mol WL: pK,1 (g/mol) (a-COOH) PK 2 (a-NH2) PK 3 Amino Acid Side Chain (side chain) Glycine Alanine 2.35 9.78 9.87 CH3 CH(CH3) CH(CH3)2 CH(CH3)2 CH2CH(CH3)2 CH(CH2OH)
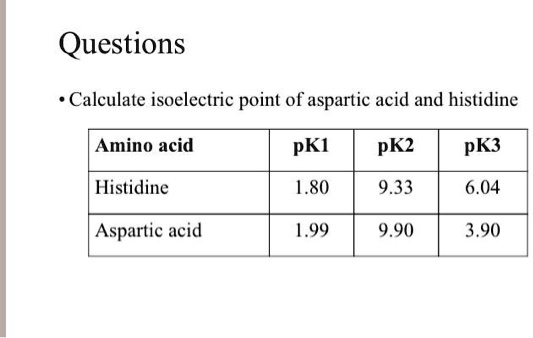
Calculate the isoelectric point of aspartic acid and histidine. Amino acid pKI pK2 pK3 Histidine 1.80 9.33 6.04 Aspartic acid 1.99 9.90 3.90