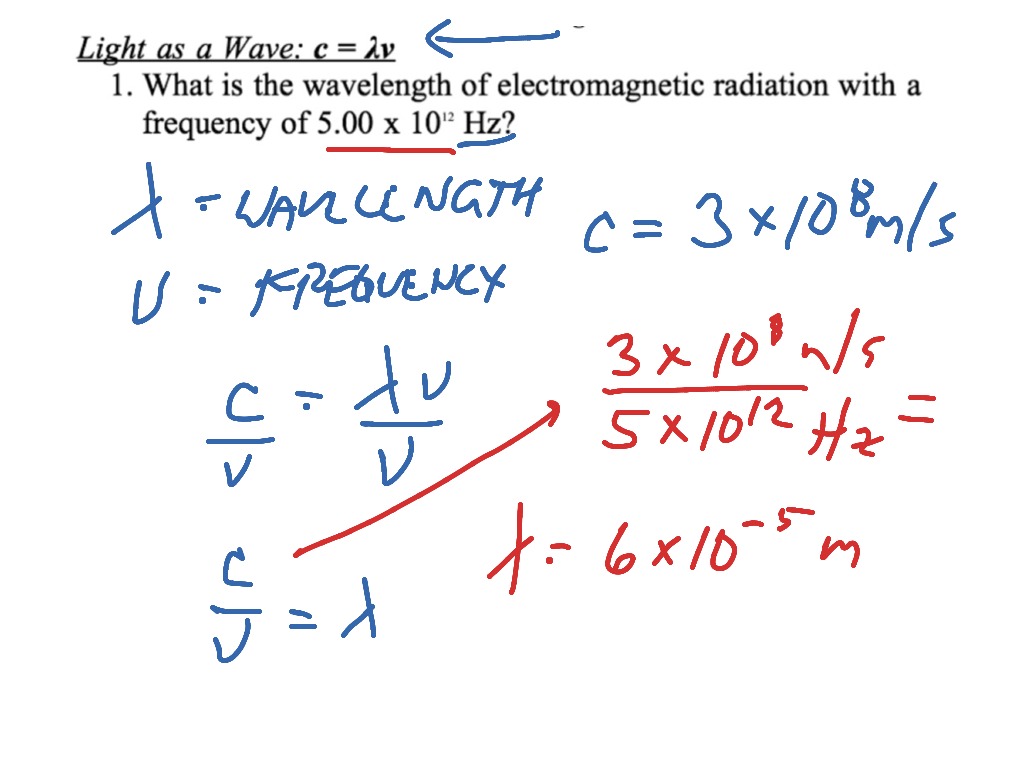
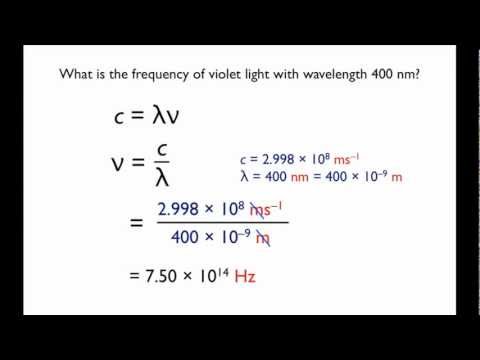
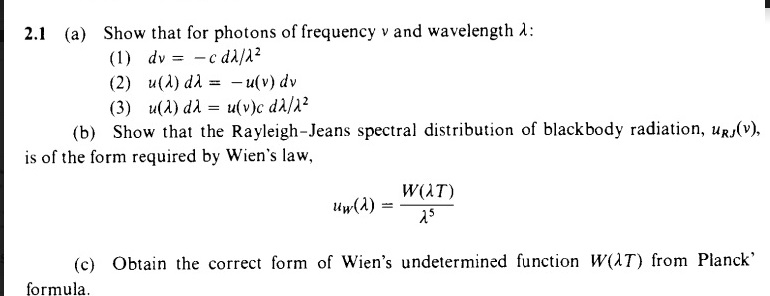
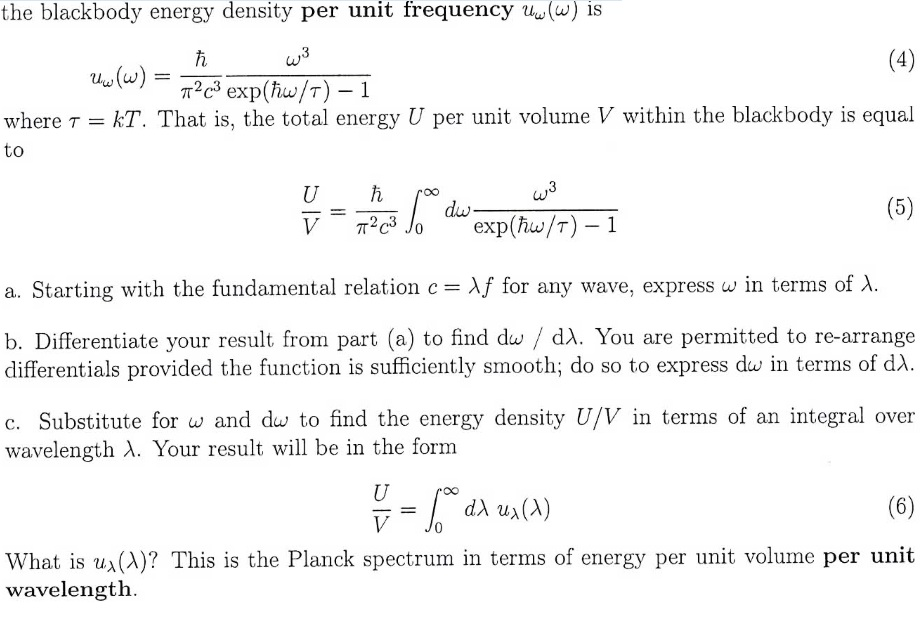
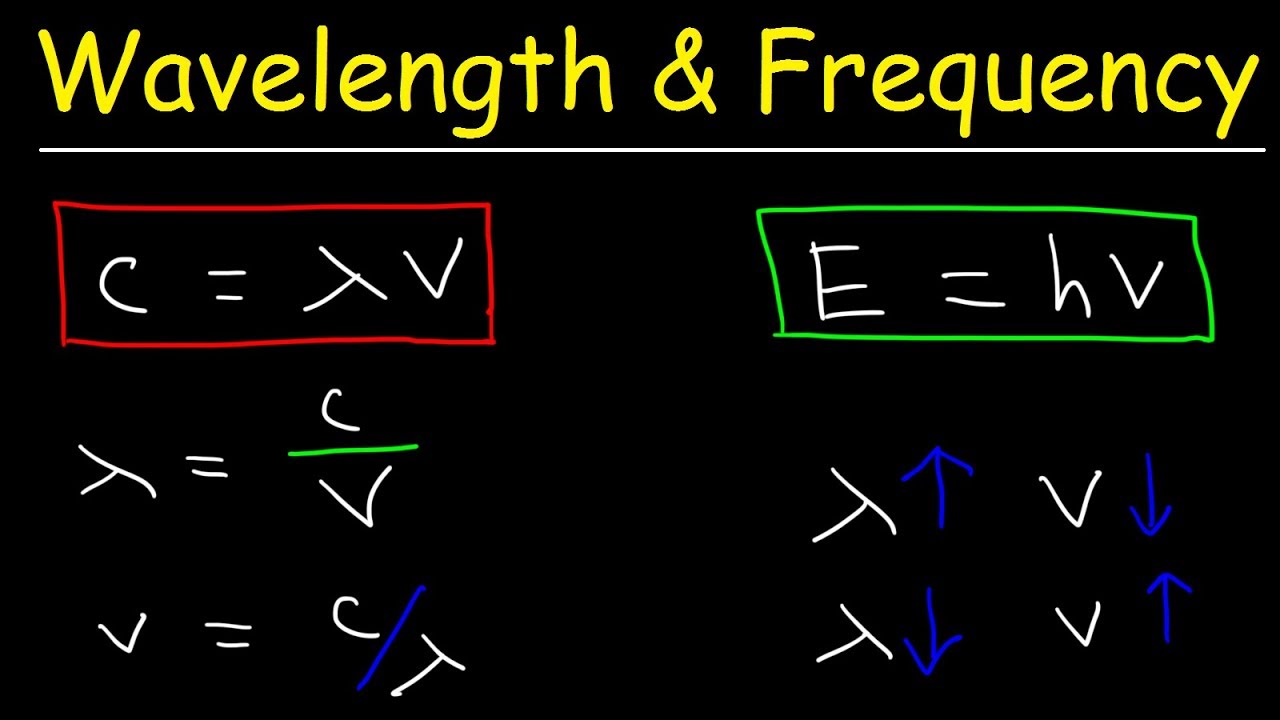
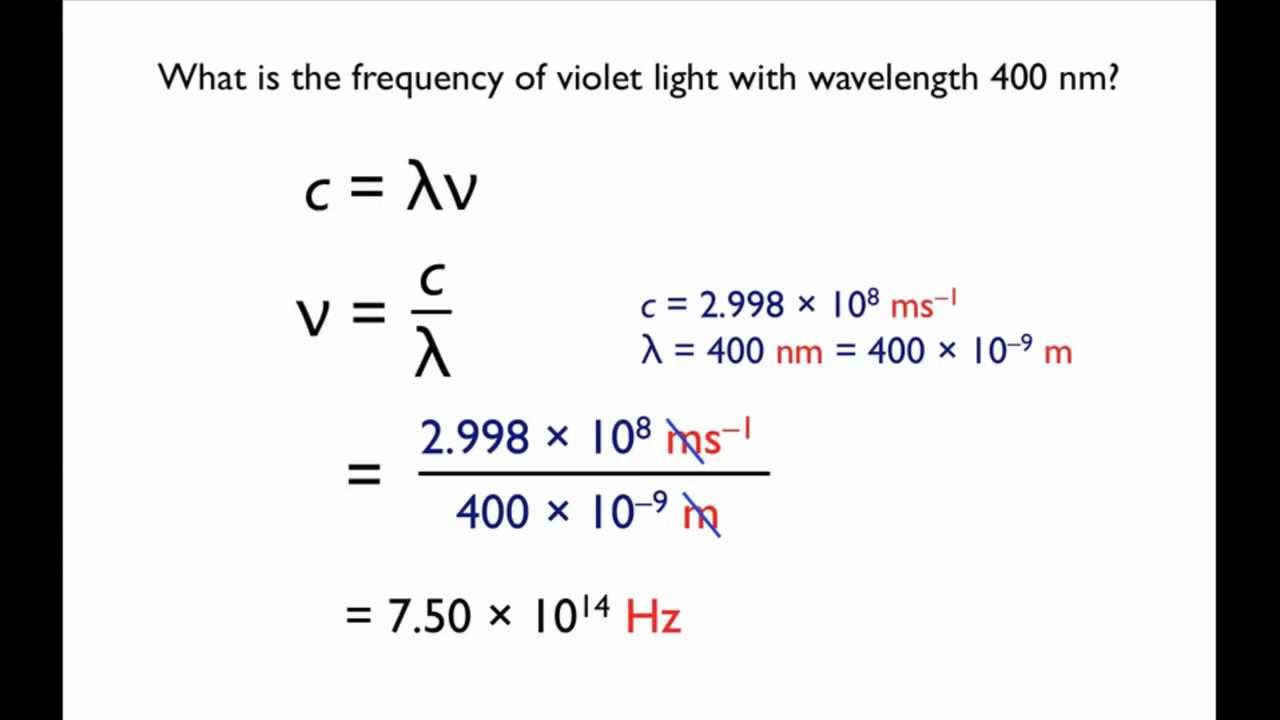
Relationship between frequency (V) wavelength (lambda) and velocity of light (c) is | CLASS 12 ... - YouTube
Using the two equations E=hv and c=lambda v derive an equation expressing E in terms of h,c and lambda.

Here's what people said they learned. 1 Difference between molecular formula and empirical formula. Welding is a physical transformation. (Mostly) It's. - ppt download
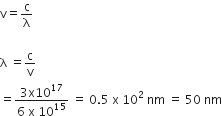
The value of Planck s constant is 6.63 x 10-34 Js. The speed of light is 3 x1017 nms-1 . Which value is closet to the wavelength in nanometer of a quantum
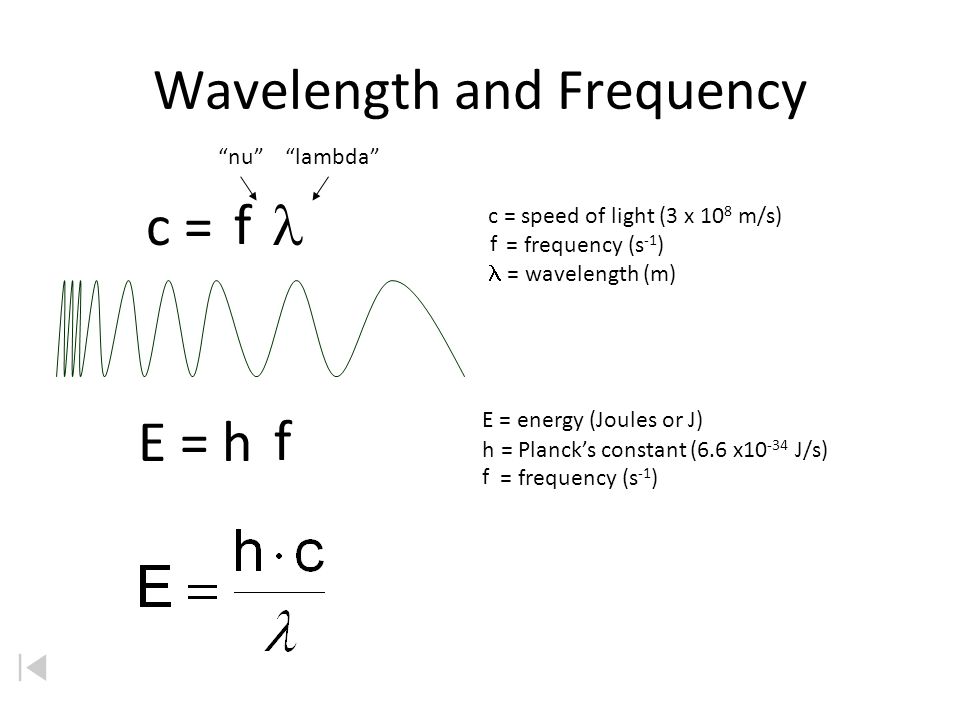
Wavelength and Frequency E = h c = c = speed of light (3 x 10 8 m/s) = frequency (s -1 ) = wavelength (m) E = energy (Joules or J) h = Planck's constant. - ppt download