Before and after the change in stability in the case where λf (0) < µc... | Download Scientific Diagram

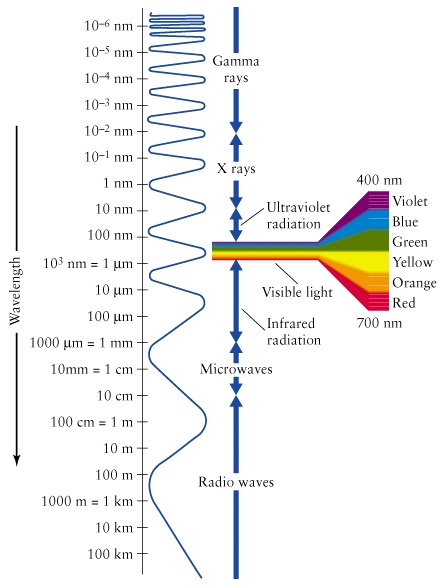
The Study of Light. The Electromagnetic Spectrum includes gamma rays, X-rays, ultraviolet light, visible light, infrared radiation, microwaves, and. - ppt download

Fluorescence emission quantum distributions EF(λ) of QuasAr1 in pH 8... | Download Scientific Diagram

DIFFERENT FORMS OF ELECTROMAGNETIC WAVES | APPLICATIONS AND EFFECTS OF ELECTROMAGNETIC WAVES - YouTube